Accumulation Process
Mercury is an inorganic element that is released into the atmosphere through man-made (mining, manufacturing processes, coal-fired utilities, or industries) or natural processes (volcanoes or weathering of rocks). It falls out of the air by sticking to dust particles or gets washed out by rain or snow and then runs off into streams and lakes. When moving through the environment, mercury goes through a series of complex changes. When these changes occur in lake and river sediments, some of the mercury can be converted to an organic form – methyl mercury. Methyl mercury enters the food chain and accumulates most readily in predator species of fish. It can then be passed to people who eat these fish.
PROCESSES
When harmful emissions, from various industry and natural sources, that contain mercury volatilize into the atmosphere or deposit into the earth, they can be released through two process; wet deposition and dry deposition. Both of these processes occur on a local, regional, and global bases. Some deposition can even occur from sources thousands of mile away.
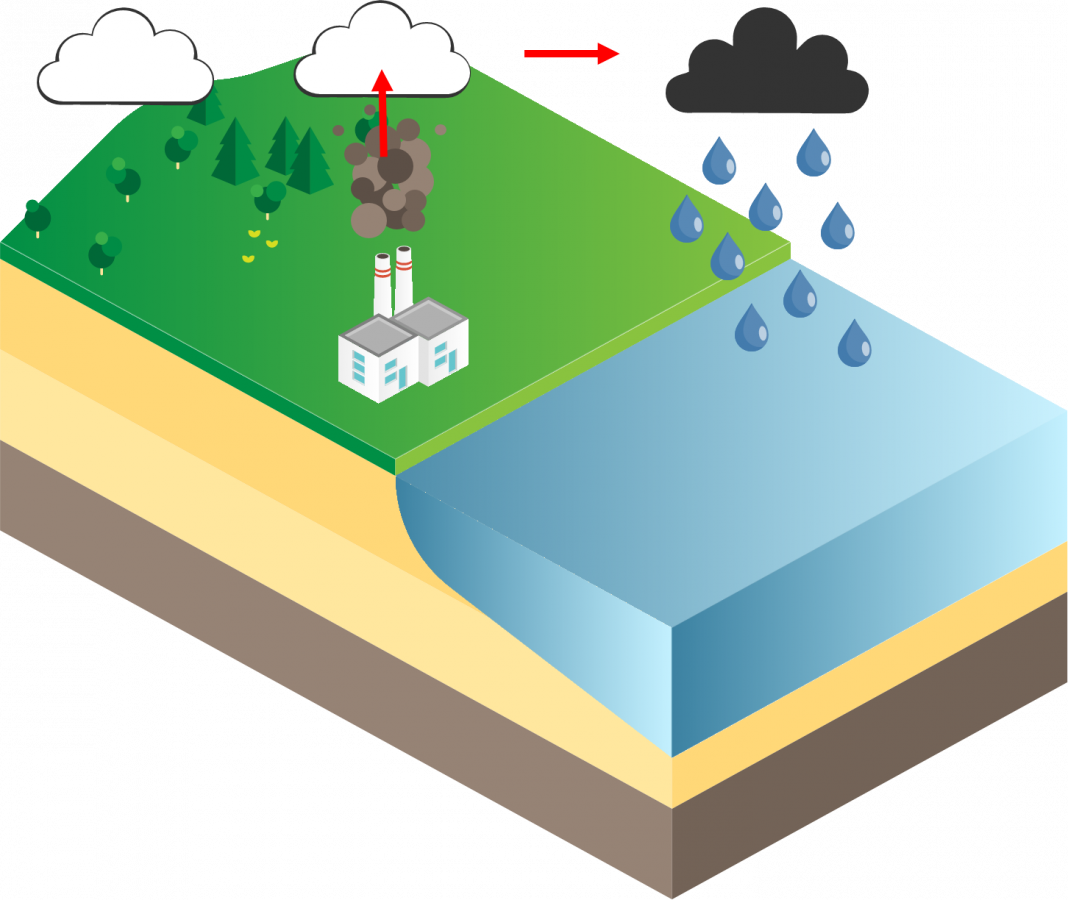
Wet deposition is when the mercury absorbs in the cloud’s droplets and then is released by precipitation (e.g. rain, snow). This can happen directly over bodies of water or in rivers where it will then accumulate in bodies of water.
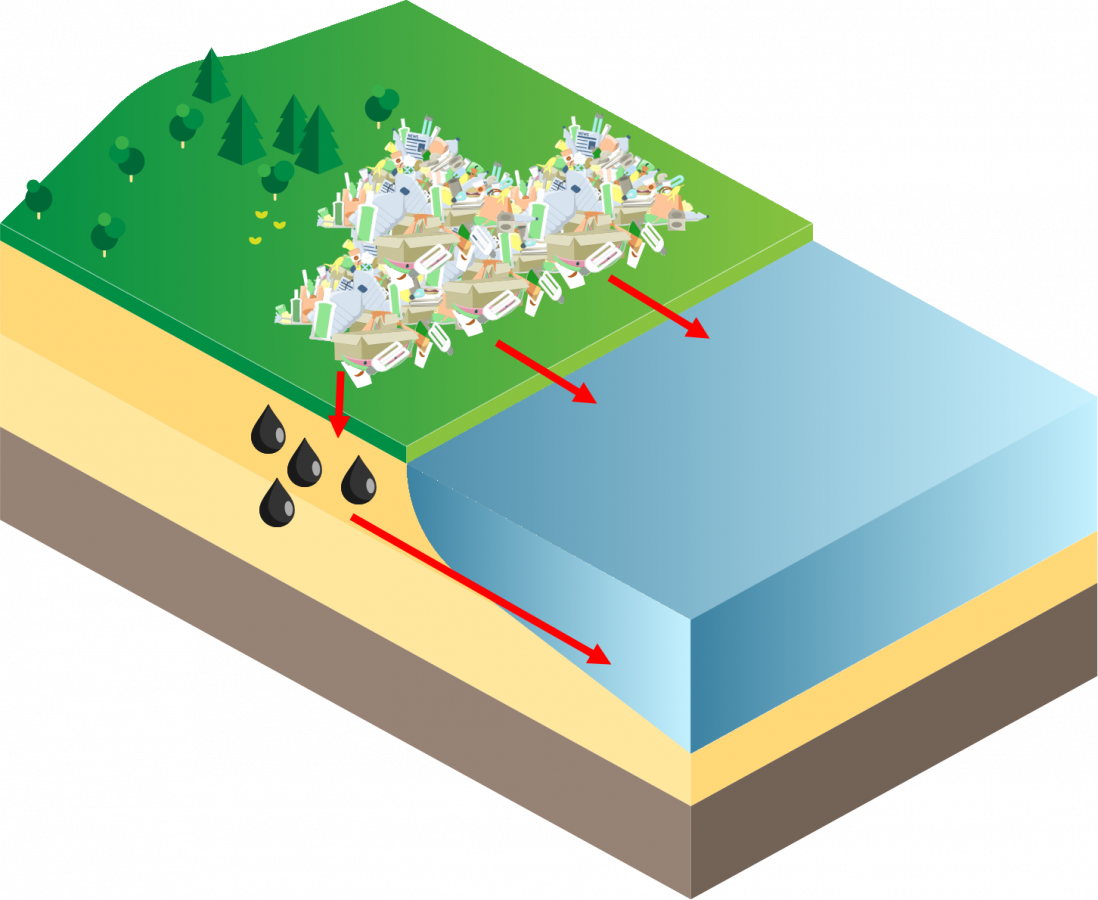
Dry deposition is when mercury attaches to particles (e.g. dust, smoke) and travel throughout the atmosphere before depositing on the earth’s surface. Mercury can then enter waterbodies through runoff and leaching.
Industrial
- Mining
- Coal-fired Energy Plants
- Municipal Waste Incinerators
- Sewerage Releases
- Landfills
- Medical Waste Incinerators
Natural
- Volcanic Activity
- Biomass Burning
- Weathering of Rock
METHYLMERCURY
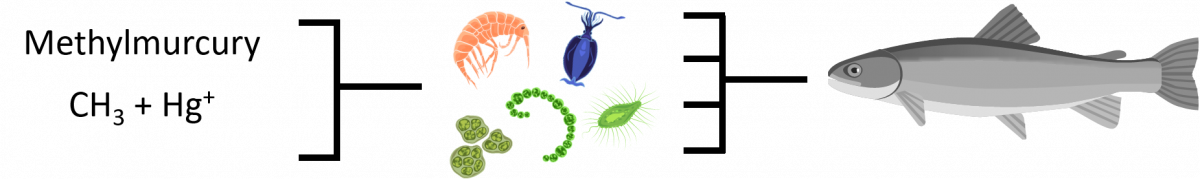
Once mercury has entered the water body or soil, it is converted into a highly toxic form called methylmercury through natural, biogeochemical transformations. Methylmercury absorbs into the body about six times more easily than inorganic mercury and can migrate through cells which normally form a barrier to toxins. For fish, methylmercury holds tightly to fish protein when absorbed through the gills or when contaminated food sources are eaten. In general, levels of mercury increase with fish size and age, but not always. Levels in fish vary by species and even lake location due to transformation rates and mercury inputs from wet and dry deposition.
BIOACCUMULATION
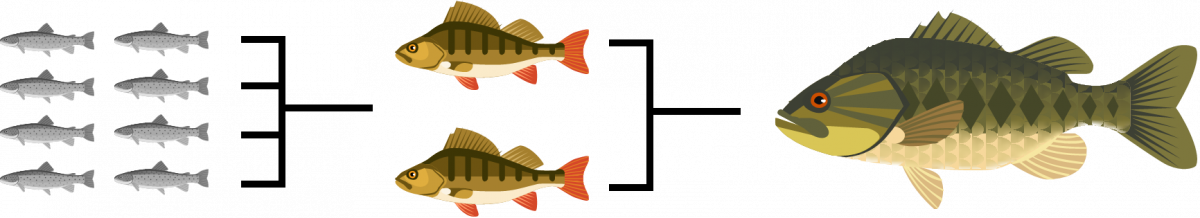
Bioaccumulation is when the zooplankton (tiny animals that serve as a food source for small fish) take in methylmurcury from the sediment at the bottom of the lake.
BIOMAGNIFICATION
When small fish begin ingesting the zooplankton that has accumulated methylmercury, they begin to accumulate the methylmercury themselves. When a bigger fish eat numerous infected fish, they are accumulating more at a faster rate.