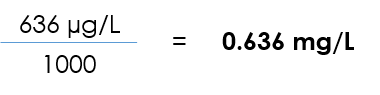Test Result Interpretation
On all laboratory reports you will see a variety of units and symbols. It is important to understand what these symbols are and what they mean for result interpretation. You may be required to convert your symbols and understanding what they are will make that process easier. The chart below are the common (but not all) units the SELS uses for reporting.

UNITS & CONVERSIONS
ppm: Parts Per Million
The number of parts of solute per 1 million parts of total solution. This can be applied to liquid and solid measurement.
Also Known As: mg/L; mg/kg; μg/g
In one Olympic size swimming pool, 1 ppm equals 1.25 liter bottles
Example: 5 ppm of lead
5 grams of lead in 1 million grams of solution OR 5 milligrams of lead in 1 million milligram of solution OR 5 pounds of lead in 1 million pounds of solution
ppb: Parts Per Billion
The number of solute per 1 billion parts of total solution.This can be applied to liquid and solid measurement.
Also Known As: μg/L; μg/kg
In one Olympic size swimming pool, 1 ppb equals 0.5 teaspoon
Example: 5 ppb of lead
5 grams of lead in 1 million grams of solution OR 5 milligrams of lead in 1 million milligram of solution OR 5 pounds of lead in 1 million pounds of solution
μg/g: Microgram Per Gram
Also Known As: ppm
mg/kg: Milligram Per Kilogram
Also Known As: ppm
μg/kg: Microgram Per Kilogram
Also known As: ppb
Helpful Conversions
- μg/g → mg/kg
- 1 μg/g = 1 mg/kg
mg/L: Milligram Per Liter
Also known as: ppm
μg/L: Microgram Per Liter
Also known as: ppb
Helpful Conversions
- μg/L → mg/L
- n μg/L ÷ 1,000 = mg/L
- mg/L → μg/L
- n mg/L x 1,000 = μg/L
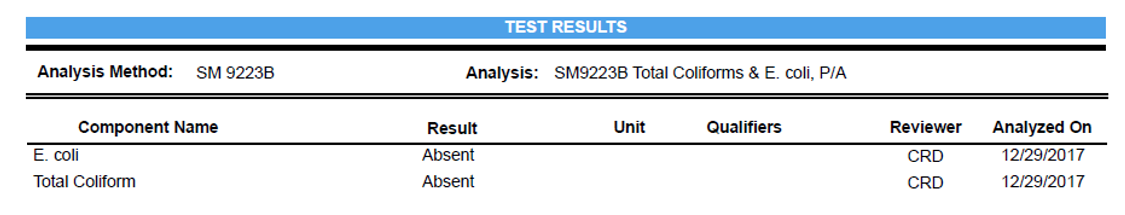
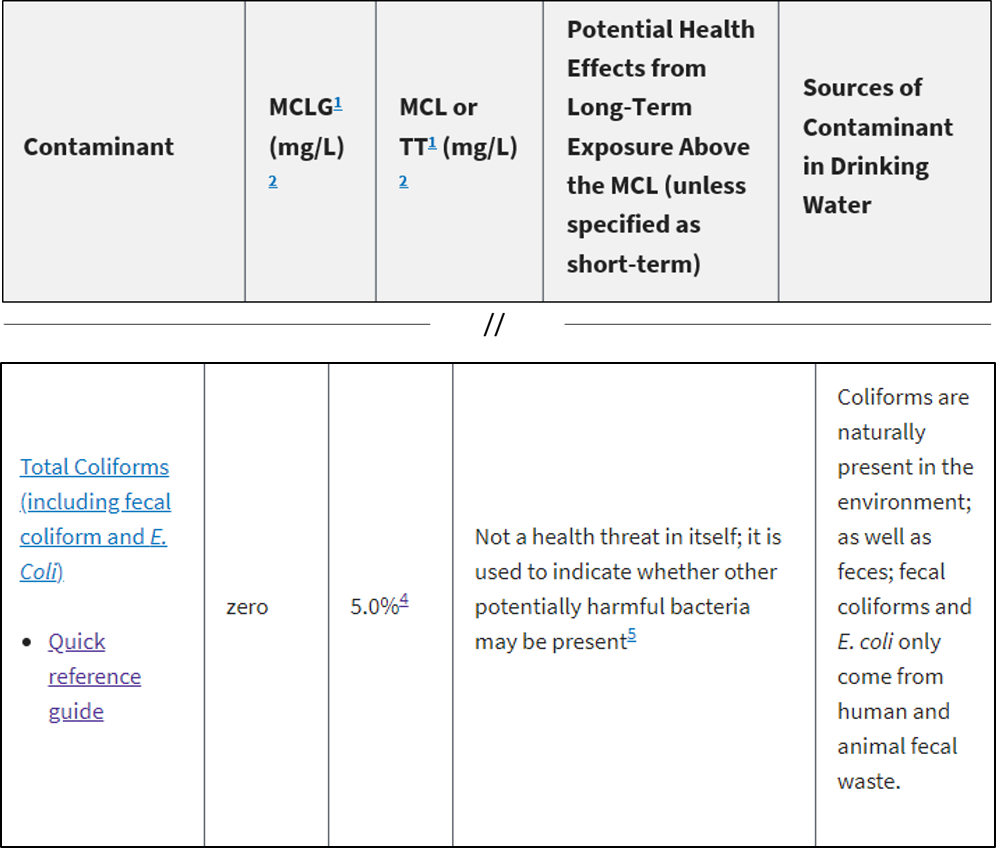
MPN: Most Probable Number
Estimate of concentration of viable microorganisms in a sample by means of replicate liquid broth growth in ten-fold dilutions.
Example: 50 MPN/100 mL
50 MPN/100 mL means that the Most Probable Number of viable cells in 100 mL of sample is 50
Present or Absent
The presence or absence of microorganisms
Also known as: P/A OR +/-
For public water supplies’ drinking water (water that has been chemically treated). By law, EPA states that the presence of any bacteria in drinking water must be treated (regardless of bacteria quantity).
CFU: Colony Forming Units
Number of viable bacterial cells in a sample per unit of volume
Example: 50 CFU/100mL
50 CFU/100 mL means 50 Colony Forming Units per 100 mL of sample
pCi: Picocuries
The curie is a standard measurement for the intensity of radioactivity contained in a sample. It’s not a measurement of volume or mass like in liquids or solids but the measurement of decay of radioactive atoms per minute. The basis for the curie is radioactivity of one gram of pure radium. Radium decays at a rate of apporxiametly 2.2 trillion disintegrations per minute. A picocurie is equal to the decay of two radioactive atoms per minute.
Example: 4 pCi/L
4 pCi/L means 8 atoms decaying every minute in every liter of water
DPM: Disintegrations Per Minute
The number of atoms that decay in one minute
C: Celsius
Measurement of temperature
0 °C is the freezing point of water
100 °C is the boiling point of water
Helpful Conversion
- Celsius to Fahrenheit:
- (n °C x 1.8) + 32
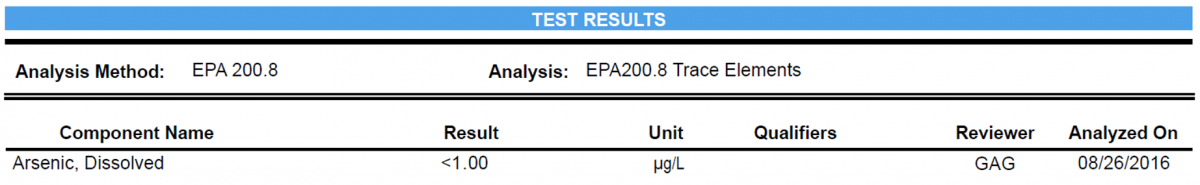
< : Less Than
Denotes a value being smaller than the number in comparison.
When this symbol appears on a SELS report, it means the value was less than the analytical reporting limit.
J Qualifier
Indicates an estimated value
MI Qualifier
Indicates a matrix interference and results could be biased high or low
HT Qualifier
Indicates hold time was exceeded before sample receipt or sample analysis
IP Qualifier
Indicates sample was improperly preserved
BR Qualifier
Indicates sample was received broken
LA Qualifier
Indicates a lab accident occurred and the sample can’t be analyzed
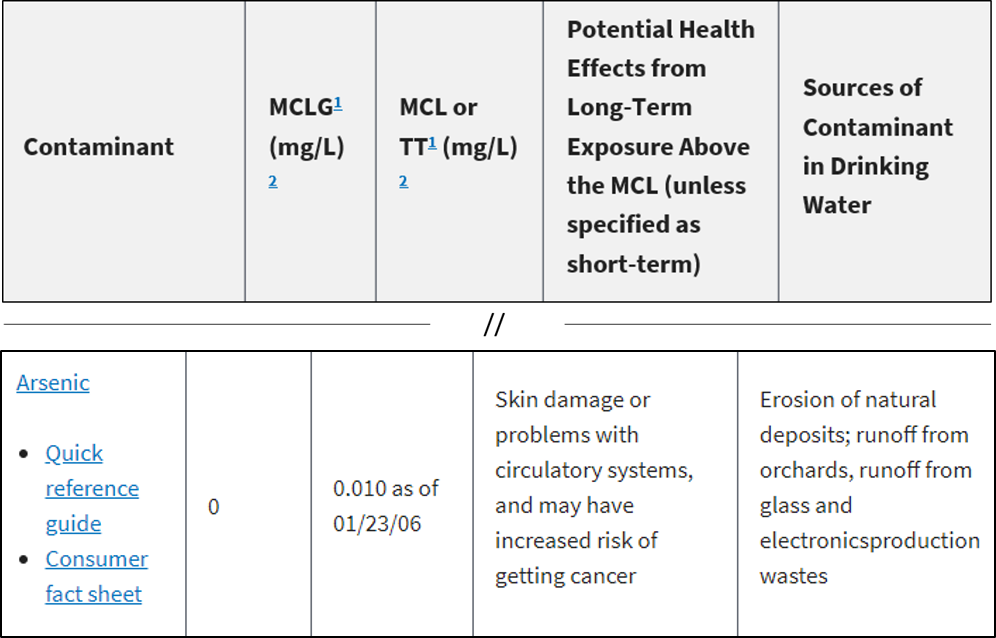
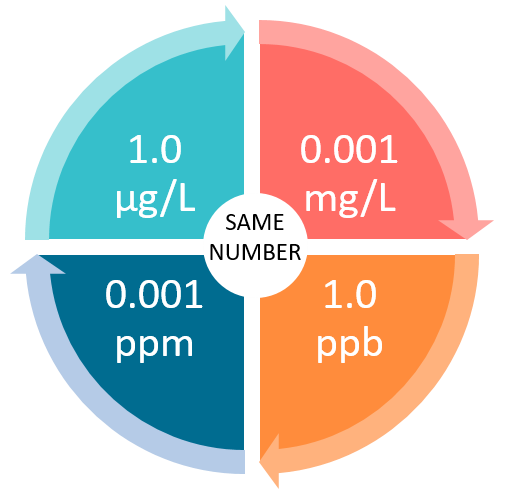
Even though some units look different, they can actually be equivalent. This can get confusing when you begin comparing different laboratory reports or looking at EPA’s MCLs. Be prepared to convert and understand what units are equivalent.
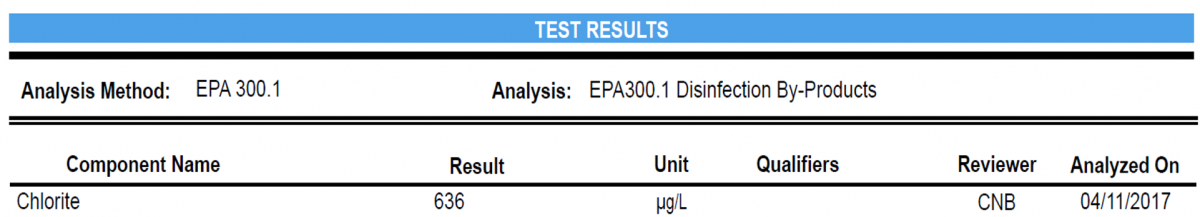
Example: Result is 6 mg/l
6 mg/L = 6 ppm = 6,000 ppb = 6,000 μg/L
HOW TO READ RESULTS
For concentrations of chemicals in water, they are measured in units of the mass of chemical (mg or µg) per volume of water (L). This is written as mg/L or µg/L. For example, your SEL results show 51.1 µg/L for Lead. This means for every 1 liter of water sample, there is 51.1 µg of Lead (or 0.0511 mg/L).
For concentrations of chemicals in soil samples, they are measured in units of the mass of chemical (mg or µg) per mass of soil (kg). This is written as mg/kg or µg/kg. For example, your SEL results show 51.1 µg/kg for Lead. This means for every 1 kilogram of soil sample, there is 51.1 µg of Lead (or 0.0511 mg/kg).
TEST RESULTS
Understanding your results is an easy three step process;
- Compare
- Convert (if applicable)
- Conclude
NEED A LITTLE MORE ASSISTANCE
Still unsure about your results? Give us a call at 405-702-1000 or email us at selsd@deq.ok.gov
Make sure you have your results on hand when contacting us. Below is the information you will need in order for us to better serve you.
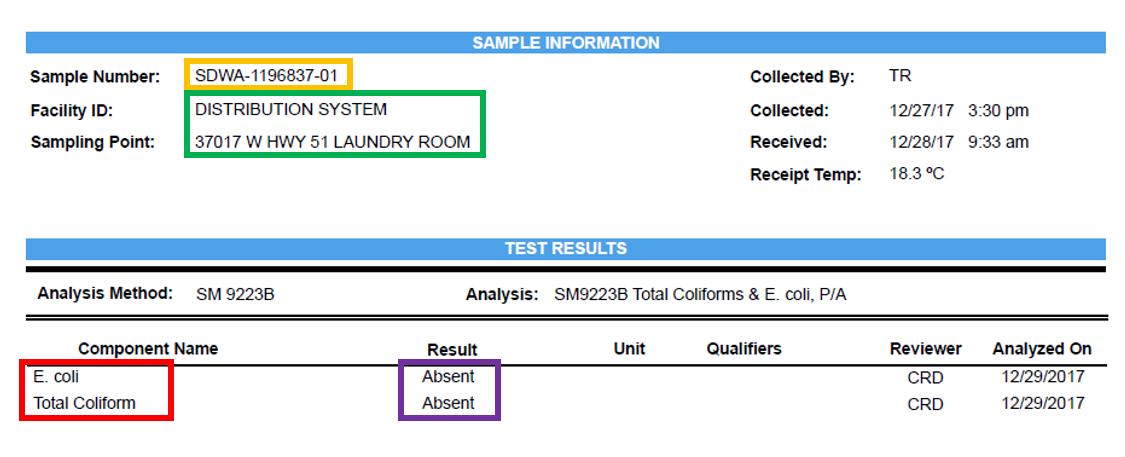
- Sample Number (yellow): This is a unique ID that SELS gives to your specific sample
- Facility ID & Sampling Point (green): For Public Water Supplies only
- Component Name (red): This is what your sample was analyzed for
- Result (purple): What your analysis concluded